About Us
The Division of Environmental Geosciences investigates key processes controlling the natural environment and anthropogenic impacts. Researchers at EDGE combine field observations with experimental work, linking molecular-scale mechanisms at environmental interfaces with complex large-scale environmental processes using quantitative modelling. At EDGE, we accept the challenges posed by the release of known and emerging pollutants and recognise the need to understand their impacts on soils, ground and surface waters, using process-based and mechanistic research.
News
03.06.2024 15:05
25.04.2024
16.04.2024
EDGE Lecture Series
27.06.2024 16:00
13.06.2024 16:00
06.06.2024 16:00
Publications
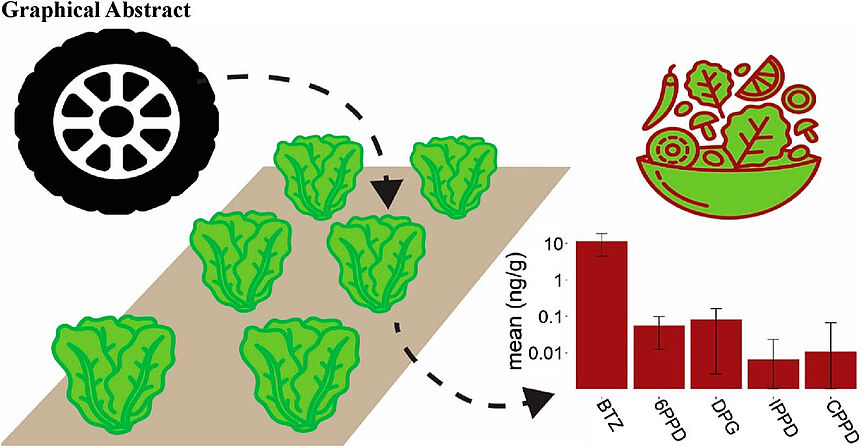
Sherman, A., Hämmerle, L. E., Ben Mordechay, E., Chefetz, B., Hüffer, T., & Hofmann, T. (2024). Uptake of tire-derived compounds in leafy vegetables and implications for human dietary exposure. Frontiers in Environmental Science, 12, [1384506]. https://doi.org/10.3389/fenvs.2024.1384506
Chaudhuri, S., Sigmund, G., Kumar, N., Hüffer, T., Mautner, A., & Hofmann, T. (2024). The efficacy of Pb, As(V) and Sb(III ) removal by biochar is determined by solution chemistry. Environmental Science: Water research & technology, 10(4), 912-921. https://doi.org/10.1039/D3EW00726J
Bubl, M., Heinz, P., Wanek, W., Schagerl, M., Hofmann, T., & Lintner, M. (2024). Impact of heavy metals (Cu, Fe, Pb, Zn) on carbon and nitrogen uptake of the diatom-bearing benthic foraminifera Heterostegina depressa. Heliyon, 10(6), [e27229]. https://doi.org/10.1016/j.heliyon.2024.e27229
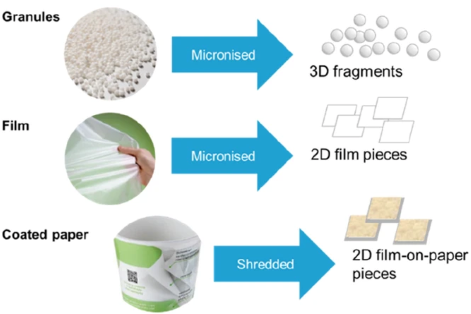
Pfohl, P., Rueckel, M., Meyer, L., Battagliarin, G., Künkel, A., Hüffer, T., Zumstein, M., Hofmann, T., & Wohlleben, W. (2024). Influence of plastic shape on interim fragmentation of compostable materials during composting. Microplastics and Nanoplastics, 4, [7]. https://doi.org/10.1186/s43591-024-00084-8
Jachimowicz, P., Peng, R., Hüffer, T., Hofmann, T., & Cydzik-Kwiatkowska, A. (2024). Tire materials disturb transformations of nitrogen compounds and affect the structure of biomass in aerobic granular sludge reactors. Journal of Hazardous Materials, 465, [133223]. https://doi.org/10.1016/j.jhazmat.2023.133223